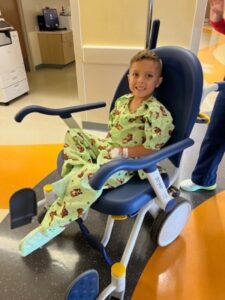
Arturo Beltran
from Chile to Dayton for Canavan disease clinical study

- patient name: Arturo Beltran
- age: 1
- condition: Canavan disease
- seen in: neurosurgery
- providers: Robert Lober, MD, PhD, FAANS
Felipe and Francisca are from the tranquil city of Valdivia in the south of Chile. It’s the city in which they got married and decided to spend the rest of their lives so they could be close to family. At least that was the plan.
On August 31, 2021, Felipe and Francisca gave birth to their son, Arturo. From the moment he was born, Felipe and Francisca knew that Arturo was special. Everything about his newborn stage was going as expected. He was growing and eating normally, and nothing would have led them to believe that Arturo was anything but a typical newborn.
When Arturo turned six weeks old, everything changed. He began to experience nystagmus several times a day. Nystagmus is a condition that causes your eyes to make rapid, repetitive and uncontrolled movements. The next day Felipe and Francisca took Arturo to the hospital in hopes of finding an answer that would help their son.
Arturo endured numerous appointments and tests, but after several weeks he still didn’t have a diagnosis. His doctors were unable to point to the cause of his nystagmus.
diagnosis of a rare disease
To continue looking for answers, Arturo and his family traveled to Santiago for more exams and genetic testing. After three months of waiting, two different genetic tests came to the same conclusion, Canavan disease.
Canavan disease is an inherited, fatal, neurological disease, characterized by the spongy degeneration of the white matter in the brain, which begins at infancy and destroys a child’s vision, speech and motor function. Currently, there is no cure for Canavan disease.
Due to the rarity of the disease and not knowing enough about it, Arturo’s doctors in Chile did not want to take his case. Felipe and Francisca asked around the entire country looking for a specialist who would take Arturo’s case only to discover that he was the first case of the disease in Chile. They were alone! The only information they were able to find about the disease to them that Arturo would only live a few years and he would never be able to sit on his own, stand up, run, walk, play, speak or eat by mouth.
“we knew there had to be someone who knew about the disease or who was working on a treatment, and we were determined to find them,” said Francisca.
finding hope
After exhausting every option they could think of, Felipe and Francisca began looking for a clinical study that could benefit Arturo. To their amazement, they found a clinical study that was recruiting patients with Canavan disease for gene therapy at Dayton Children’s Hospital. Felipe and Francisca drafted an email to the contact provided and within 15 minutes, they received a response asking to schedule a video call.
During the video call, the clinical study team reviewed Arturo’s exams. They then explained the clinical study process and invited Arturo and his family to travel to Dayton for a preliminary evaluation.
After a month and a half, and a long 24-hour drive from Chile, Arturo and his family arrived at Dayton Children’s. They were met by the neurosurgery team who impressed the family immediately.
Arturo had to undergo a series of tests that would determine if he would be one of only eight children who would ultimately qualify for the initial phase I trial.
After returning to Chile, Felipe and Francisca had a follow-up video call with the clinical study team, where they learned that Arturo had qualified to participate in the study.
“we knew that this gene therapy was not yet an official cure for Canavan disease and the road ahead would be long”, said Francisca. “However, we did not hesitate to participate. We were hopeful that our son’s quality of life would improve, and his participation would contribute to making this therapy safe and effective for other children in the future.”
gene therapy in Dayton
On August 18, 2022, Arturo became the youngest person to receive the clinical application of a newly modified recombinant Adeno-Associated-Viral Vector (rAAV). The novel rAAv is customized to express a healthy gene in the myelin-producing cells to restore enzymatic function in the most needed cell compartment (white matter) and promote brain development.
Since having gene therapy, Arturo is doing well. His disease seems to be more controlled and he seems more comfortable.
Prior to receiving the gene therapy, Arturo didn’t interact or smile at his parents. Now, he smiles as a form of communication letting Felipe and Francisca know he wants to play. They feel like Arturo is more present and enjoys life more than he did before gene therapy.
Not every day is good, but most of the time, Arturo is a happy child!
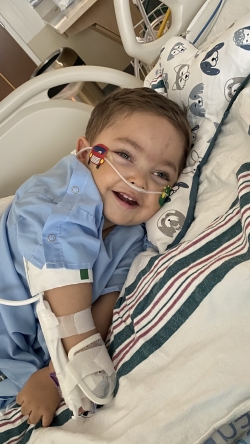
“we are so grateful for the team of professionals at Dayton Children’s”, says Francisca. “They have been genuinely interested in not only the well-being of our son but my husband and me. They support us, understand us, listen to us and are always willing to learn about our son’s disease.”
Both Felipe and Francisca will tell you that their life has not been easy, but it has been beautiful. They have been able to love Arturo in a way that is difficult to explain. Knowing that he may not be in this world for a long time has given them the strength to love him harder and stronger than they could have ever imagined.
For other families going through similar situations, Felipe and Francisca would tell you that you are not alone, as they once believed. They have found a community among other families through the clinical study experience and being able to share their experiences and support each other has provided comfort during their journey with Canavan disease.
planting roots
Given the exceptional care Arturo receives at Dayton Children’s and the uncertainty of the disease, Felipe and Francisca made the decision to stay and live in Dayton. This was not a decision they took lightly. It meant leaving behind their families, their careers, their home and their three dogs, but they felt and still believe that this was the best decision for their son.
As Arturo reaches the one-year time point in the phase I/II trial this summer and the study continues to collect data going forward, their family is looking for a way to establish themselves in the United States more permanently.

share your story
Every patient journey at Dayton Children’s is powerful — from NICU miracles to courageous cancer survivors and beyond. These patient stories not only celebrate our families but also offer hope and encouragement to others facing similar challenges. Share your experience today and help inspire, support, and celebrate the strength of our patients and families.
care that goes above and beyond
Because every child deserves care that goes above and beyond, Dayton Children’s provides compassionate, expert care for kids of all ages. Find a provider, schedule an appointment, or learn more about conditions we treat today.